Molecular Simulation of Phase Equilibria
Start date: 2003-01-01
End date: 2009-12-31
Traditionally, phase equilibrium data has been obtained by experimental measurement. Experimental measurements are, however, extremely time-consuming and expensive. Just one pair of data points can often cost several hundred dollars. In addition, experimental measurement is often hampered by the fact that the system under investigation may be prohibitively difficult to measure (e.g. high temperature or high pressure) or composed of hazardous or toxic chemicals. A good understanding of the behaviour of a system usually requires a large number of data sets at a variety of isobars or isotherms.
The other major impediment to the purely experimental approach arises because of the possible permutations of chemical systems. There are an estimated 6 million known chemical compounds with thousands of new chemical species appearing each year. Adequate pure component thermodynamic data exists only for a few thousand of these chemicals. The situation worsens when one considers the case for binary mixtures and becomes nearly impossible when considering ternary or higher mixtures and the possible combinations of composition, pressure and temperature. Clearly, any attempt to systematically accumulate some kind of comprehensive phase equilibrium thermodynamic library by purely experimental means will fall drastically short. The awesome size of the task implies that the idea of experimental measurement merely keeping pace with the generation of new chemical species will not be possible.
Molecular simulation offers the possibility of being able to generate thermodynamic data as it is required efficiently, cost effectively and accurately and it is with his in mind that the current project has been undertaken. In this project the Configurational-bias Monte Carlo method in the Gibbs Ensemble is being used to simulate phase equilibrium data for a variety of systems.
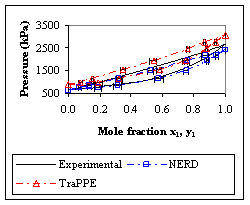
An example is the ethane + propene binary mixture. As illustrated in Fig. 1., the simulated data yielded the same trends as experiment, and in some cases are in quantitative agreement. This is an important result when using simulated data for designing industrial equipment, because it suggests that phase equilibrium data from molecular simulations can potentially provide reliable compositional information for sizing separation vessels and that the industrial use of simulated compositions for mixtures of hydrocarbons is not limited by the need for more sophisticated force fields.
A joint project between University of Borås and University of KwaZulu Natal (Durban)
Project Leader
Kim Bolton
Professor
033-435 4602


